Abstract
Introduction: Proteasomes are the chief degradation components of the ubiquitin proteasome system (UPS) and are comprised of several (𝜶) structural and (ß) catalytic subunits. Targeting the ß5 subunit, with agents such bortezomib (Btz, 20S proteasome inhibitor, PI) has proven highly successful in MM pts. However, resistance to Btz (Btz-R) invariably ensues with disease evolution resulting in relapse and mortality. Our data highlight that in Btz-R cells, proteasome function is upregulated with increased reliance on other ß-catalytic subunits. We have uncovered a novel association between the ß5 subunit acting in concert with the ß1i proteasome subunit, to promote resistance to Btz. We hypothesized that targeting ß5 and ß1i together would be lethal to Btz-R MM cells.
Methods: CD138+ cells from MM patients, Btz-sensitive (Btz-S) MM cell lines (OPM2, KMS11 and U266) and their isogenic Btz-R subclones were used in experiments. Fluorogenic peptide substrates were used to measure ß-subunit-specific enzymatic activity. Gene disruption was conducted using 2 different shRNA hairpins for PSMB5 (ß5) and PSMB9 (ß9). Affymetrix HT12v4 gene expression array and NanoString mRNA quantification were used for gene expression profiling (GEP), followed by qPCR for confirmation. Novel dual ß5-ß1i PI were synthesized and tested in vitro. Apoptosis was determined by annexin-V/PI staining and MTS/CellTiter Glo assay used to determine cell viability.
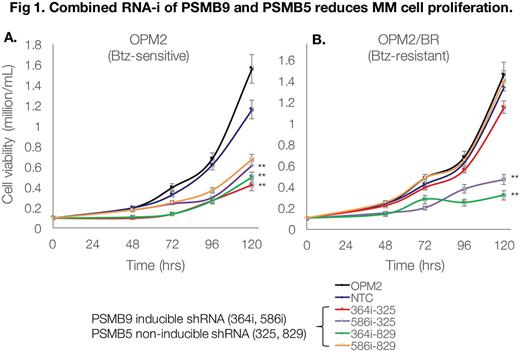
Results: Btz IC50 was noted to be 284nM in Btz-R vs. 4nM in Btz-S cells. Sanger seq. revealed no mutations in PSMB5 indicating that Btz should effectively bind the ß5 subunit. ß-subunit chymotrypic activity in Btz-R vs. Btz-S cells was significantly increased (3.5 fold, p<0.001) but remained amenable to downregulation with Btz (as well as other PI). This suggested that ß5-subunit-independent mechanisms may account for increased proteasome function and resistance to Btz. GEP analysis revealed modulation of several genes; in particular, PSMB9 (ß1i) in Btz-R vs. Btz-S MM cells. Proteomic analysis confirmed protein/enzymatic upregulation of PSMB5/ß5 in conjunction with PSMB9/ß1i in primary cells from Btz-R MM patients and Btz-R MM cell lines. Clinical significance of PSMB9 upregulation was queried using the MMRF CoMMPass database (IA10), with analysis in pts. who received either Btz+dex or Len+Dex and did not respond to treatment. PSMB9 mRNA was significantly upregulated in Btz+Dex non-responders (n=42) vs. responders (n=92), whereas in Len+Dex pts. this difference was not significant. We hypothesized that downregulation of PSMB9 would resensitize Btz-R cells to Btz and noted a significant shift in IC50 (235nM to 108nM) in Btz-R-PSMB9 shRNA transfected cells. While ß1i knockdown alone was able to reduce Btz-R cell viability by ~25%, we posited that concurrent disruption of ß1i and ß5 would significantly reduce cell viability and proliferation. Targeted GEP in dual-ß5/ß1i knockdown Btz-R cells, showed >2-fold increase in CASP8, CASP9, CASP10, CDKN2A, CDKN1A , TNFRSF10B, FOXO4 and RNF43 (apoptosis genes) and decrease in CCB1, CCNA2, WNT5A, SKP2, CDC6 and FEN1 (cell cycle genes) vs. scramble-transfected controls. Indeed, both Btz-S and Btz-R MM cells demonstrated significantly compromised growth capacity and viability (Dual knockdown ß5/ß1i Btz-R cells: 0.32 million/mL vs. scramble Btz-R cells: 1.6 million/mL) (Fig 1). This prompted us to develop novel dual-ß5 and ß1i chemical inhibitors. Using a highly modified carbamate scaffold we developed 20 cmpds that display binding affinity for both ß5 and ß1i. In vitro screening of the top 5 cmpds showed >2 fold lower EC50 in Btz-R vs. Btz-S MM cell lines. 2 cmpds in particular showed ß1i and ß5 enzymatic inhibition of >50% at 1uM and 5uM, respectively and are being further optimized.
Conclusions: We have found that upregulation of PSMB9/ß1i and PSMB5/ß5 is associated with resistance to Btz. These findings were confirmed in both MM cell lines as well as in primary MM cells from Btz-R pts. Moreover, increased PSMB9 mRNA expression in non-responding Btz+Dex pts. but not Len+dex MM pts. highlights this as a resistance mechanism unique to Btz. Direct genetic disruption or with novel dual-chemical inhibitors directed toward ß1i and ß5 induced lethality in Btz-R (as well as Btz-S) MM cells. Thus, our investigations point to development of agents, which inhibit ß1i and ß5-proteasome subunits to overcome resistance to Btz.
Ailawadhi: Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Research Funding; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal